Orbital with the following boundary-surface representations uncovers the intricate world of atomic orbitals, delving into their shapes, properties, and significance in chemistry and physics. These boundary-surface representations provide a visual framework for understanding the complex behavior of electrons within atoms and molecules.
Through a comprehensive exploration of orbital energy levels, hybridization, and applications, this discourse illuminates the fundamental principles governing atomic and molecular structure. By delving into advanced concepts such as spin-orbit coupling and relativistic effects, we gain a deeper appreciation for the intricate tapestry of quantum mechanics.
Orbital Definition
In astronomy, an orbital refers to the curved path that a celestial body follows as it revolves around another celestial body, such as a planet around a star or a moon around a planet. The shape of an orbit is determined by the gravitational forces acting between the two bodies, and it can vary from being nearly circular to highly elliptical.
Orbitals are characterized by several key elements, including:
- Eccentricity:This parameter describes how elliptical an orbit is, with a value of 0 indicating a circular orbit and values closer to 1 indicating a more elliptical orbit.
- Semi-major axis:This is the average distance between the two orbiting bodies.
- Orbital period:This is the time it takes for the orbiting body to complete one full revolution around the central body.
- Inclination:This parameter measures the angle between the orbital plane and a reference plane, such as the ecliptic plane in the Solar System.
Types of Orbitals
Orbitals can be classified into different types based on their shape and energy level. The most common types of orbitals are:
- s-orbitals:These are spherical orbitals that have no angular momentum and are centered on the nucleus.
- p-orbitals:These are dumbbell-shaped orbitals that have one unit of angular momentum and are oriented along the x, y, or z axes.
- d-orbitals:These are more complex orbitals that have two units of angular momentum and come in five different shapes.
- f-orbitals:These are even more complex orbitals that have three units of angular momentum and come in seven different shapes.
Orbital Representations
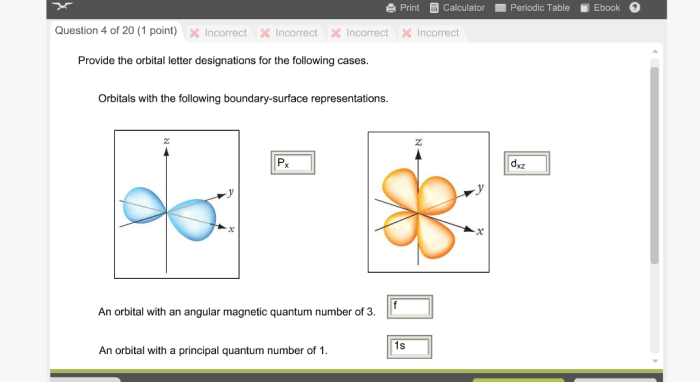
There are various boundary-surface representations used to describe orbitals. These representations provide a visual representation of the probability of finding an electron in a particular region of space.
Some of the most common orbital representations include:
- Electron density plots:These plots show the probability of finding an electron at a given point in space.
- Contour plots:These plots show the surfaces of constant electron density.
- Radial distribution functions:These plots show the probability of finding an electron at a given distance from the nucleus.
Orbital Energy Levels
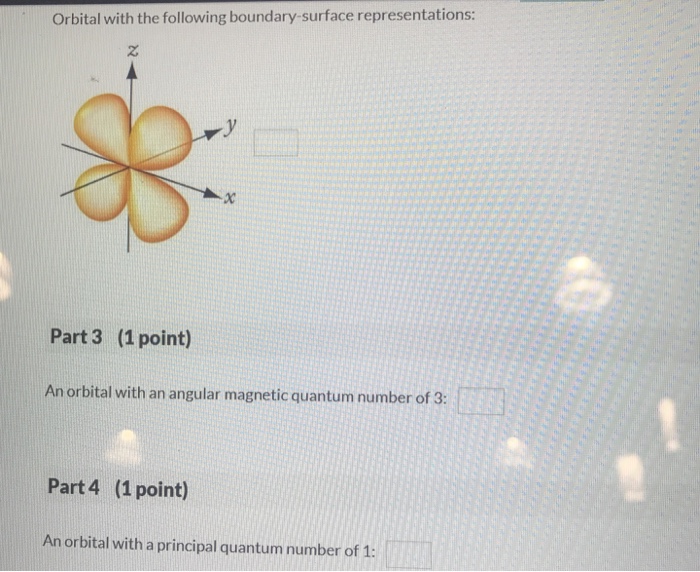
Orbitals have specific energy levels associated with them. The energy level of an orbital is determined by its shape and the distance from the nucleus. Orbitals with lower energy levels are closer to the nucleus and have a higher probability of finding an electron.
The energy levels of orbitals are quantized, meaning that they can only exist at certain discrete values. The energy levels of orbitals are typically represented using the principal quantum number (n), the angular momentum quantum number (l), and the magnetic quantum number (ml).
Orbital Hybridization
Orbital hybridization is a process in which atomic orbitals combine to form new hybrid orbitals that have different shapes and energies than the original orbitals.
Hybridization occurs when atoms form covalent bonds. The type of hybrid orbitals that form depends on the number and type of atomic orbitals that are involved in the bonding.
Some of the most common types of hybrid orbitals include:
- sp hybridization:This type of hybridization occurs when an s orbital and a p orbital combine to form two sp hybrid orbitals.
- sp2hybridization: This type of hybridization occurs when an s orbital and two p orbitals combine to form three sp 2hybrid orbitals.
- sp3hybridization: This type of hybridization occurs when an s orbital and three p orbitals combine to form four sp 3hybrid orbitals.
Applications of Orbital Theory
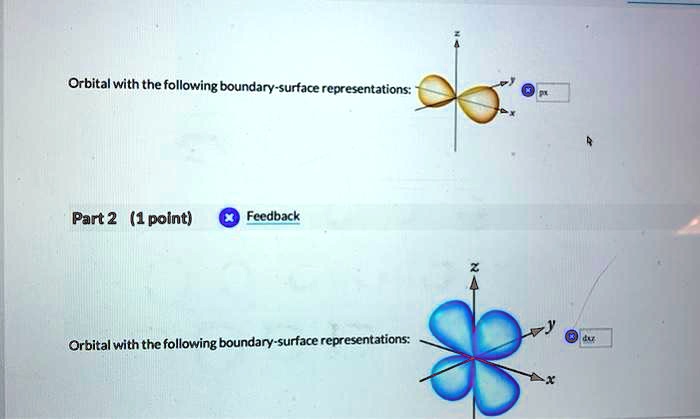
Orbital theory is a fundamental concept in chemistry and physics. It is used to explain a wide range of phenomena, including:
- Chemical bonding:Orbital theory is used to explain how atoms bond together to form molecules.
- Molecular structure:Orbital theory is used to predict the shape and properties of molecules.
- Spectroscopy:Orbital theory is used to interpret the spectra of atoms and molecules.
- Magnetic properties:Orbital theory is used to explain the magnetic properties of materials.
FAQ Summary: Orbital With The Following Boundary-surface Representations
What are boundary-surface representations of orbitals?
Boundary-surface representations are visual depictions of the three-dimensional shapes of orbitals, providing a qualitative understanding of their spatial distribution.
How do orbital shapes influence their energy levels?
The shape of an orbital determines its energy level, with orbitals of higher energy having more complex shapes and occupying larger regions of space.
What is orbital hybridization?
Orbital hybridization is the process by which atomic orbitals combine to form new hybrid orbitals with different shapes and properties, influencing the bonding behavior of atoms.