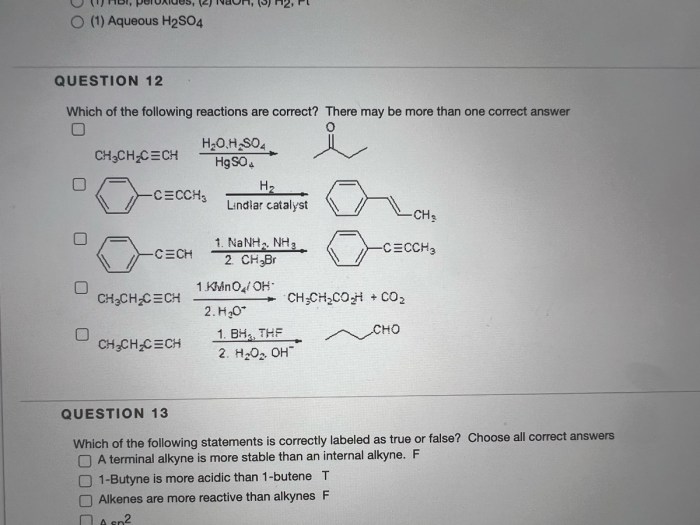
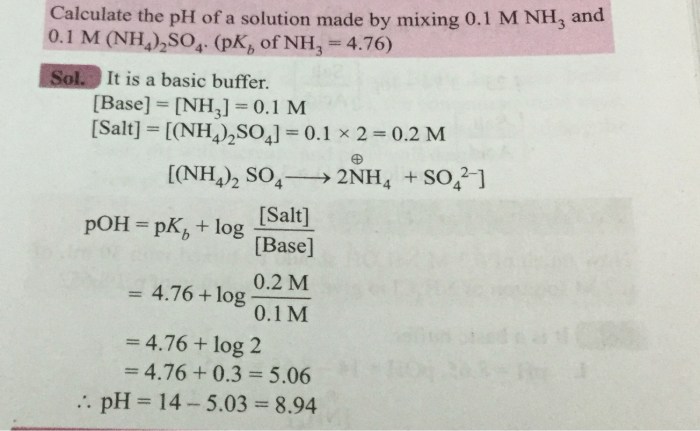Rank the following compounds from most acidic to least. – Introducing the topic of acidity, this exploration delves into the ranking of compounds based on their acidic strength. The concept of acid dissociation constants (pKa) serves as the cornerstone of this analysis, guiding us in understanding the behavior of acids in various environments.
Molecular structure plays a crucial role in determining acidity, with factors such as electronegativity, resonance, and hybridization influencing the stability of the conjugate base formed upon dissociation. Additionally, solvent polarity and temperature exert significant effects on the acidity of compounds, highlighting the dynamic nature of acid-base equilibria.
Acidity of Organic Compounds

Acidity is a measure of a compound’s ability to donate a proton (H+ ion). The stronger the acid, the more easily it donates a proton. Acid dissociation constants (pKa) are used to quantify the acidity of acids. The lower the pKa, the stronger the acid.
Acid Dissociation Constants (pKa)
The pKa of an acid is the negative logarithm of its acid dissociation constant (Ka). The Ka is the equilibrium constant for the dissociation of the acid into its conjugate base and a proton.
| Compound | pKa |
|---|---|
| Acetic acid | 4.76 |
| Benzoic acid | 4.20 |
| Phenol | 9.95 |
| Ethanol | 15.9 |
As can be seen from the table, acetic acid has a lower pKa than benzoic acid, which means that acetic acid is a stronger acid than benzoic acid. Ethanol has the highest pKa, which means that it is the weakest acid.
Molecular Structure and Acidity, Rank the following compounds from most acidic to least.
The acidity of an organic compound is influenced by its molecular structure. Factors such as electronegativity, resonance, and hybridization can all affect the acidity of a compound.
- Electronegativity: The more electronegative the atom that is bonded to the hydrogen atom, the stronger the acid. This is because the electronegative atom will pull the electron density away from the hydrogen atom, making it more likely to be donated.
- Resonance: Resonance can stabilize the conjugate base of an acid, making it less likely to donate a proton. This is because the negative charge of the conjugate base is spread out over multiple atoms, making it less concentrated and less likely to be attracted to a proton.
- Hybridization: The hybridization of the carbon atom that is bonded to the hydrogen atom can also affect the acidity of a compound. sp3-hybridized carbon atoms are more acidic than sp2-hybridized carbon atoms, which are more acidic than sp-hybridized carbon atoms.
Solvent Effects on Acidity
The acidity of an organic compound can also be affected by the solvent in which it is dissolved. Polar solvents, such as water, can stabilize the conjugate base of an acid, making it less likely to donate a proton. This is because the polar solvent molecules can solvate the conjugate base, forming hydrogen bonds with it.
Nonpolar solvents, such as hexane, do not solvate the conjugate base as well, so they do not stabilize it as much. This makes the conjugate base more likely to donate a proton, which increases the acidity of the acid.
Temperature Effects on Acidity
The acidity of an organic compound can also be affected by temperature. In general, the acidity of an acid decreases as the temperature increases. This is because the equilibrium constant for the dissociation of an acid into its conjugate base and a proton is temperature-dependent.
As the temperature increases, the equilibrium constant decreases, which means that the acid is less likely to dissociate. This results in a decrease in acidity.
Question & Answer Hub: Rank The Following Compounds From Most Acidic To Least.
What is the relationship between pKa and acidity?
pKa values are inversely proportional to acidity. A lower pKa value indicates a stronger acid, as it dissociates more readily in water to release protons.
How does electronegativity affect acidity?
Electronegative atoms, such as oxygen and fluorine, stabilize the conjugate base by withdrawing electron density away from the acidic proton, making the acid more stable and less likely to dissociate.