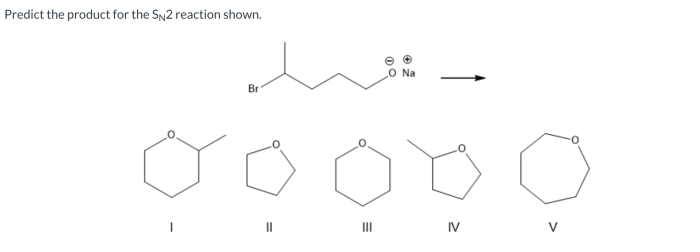
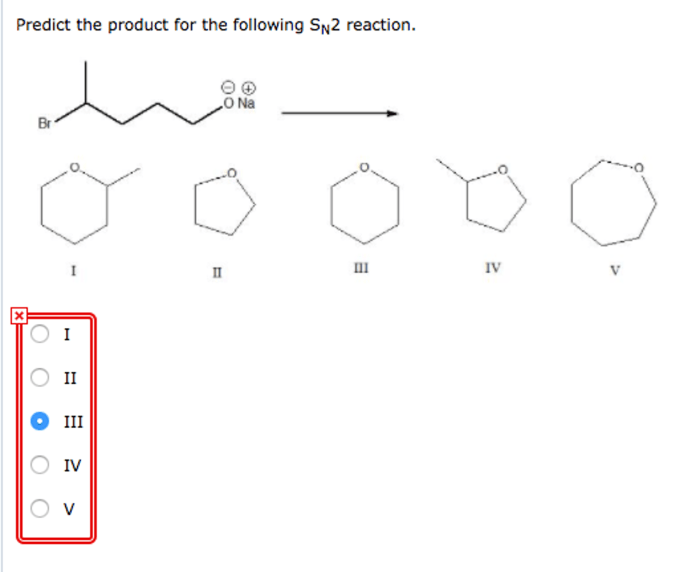
Predict the product for the SN2 reaction shown. SN2 reactions are a type of nucleophilic substitution reaction that proceeds through a concerted mechanism, meaning that the nucleophile attacks the electrophile and the leaving group departs simultaneously. This type of reaction is commonly observed in organic chemistry and is important for understanding the formation of various organic compounds.
In this guide, we will delve into the mechanism of SN2 reactions, explore the factors that affect product formation, and discuss the regioselectivity and stereochemistry of these reactions. By the end of this discussion, you will have a comprehensive understanding of SN2 reactions and be able to predict the products of these reactions accurately.
Reaction Mechanism
SN2 reactions proceed through a concerted, one-step mechanism in which the nucleophile attacks the electrophile and the leaving group departs simultaneously.
The nucleophile is a species that donates a pair of electrons to form a new bond, while the electrophile is a species that accepts a pair of electrons to form a new bond.
SN2 reactions are typically fast and occur with inversion of configuration at the reaction center.
Examples of SN2 Reactions, Predict the product for the sn2 reaction shown.
- The reaction of hydroxide ion with methyl bromide to form methanol
- The reaction of iodide ion with ethyl chloride to form ethyl iodide
- The reaction of ammonia with methyl iodide to form methylamine
Factors Affecting Product Formation

The formation of products in SN2 reactions is affected by several factors, including the strength of the nucleophile, the structure of the electrophile, and the solvent.
Effect of Nucleophile Strength
The strength of the nucleophile is a measure of its ability to donate electrons. Stronger nucleophiles are more likely to react with electrophiles to form products.
The strength of nucleophiles can be affected by several factors, including the charge of the nucleophile, the size of the nucleophile, and the presence of lone pairs of electrons.
Effect of Electrophile Structure
The structure of the electrophile can also affect the formation of products in SN2 reactions.
Electrophiles that are more substituted are less likely to react with nucleophiles because the substituents hinder the approach of the nucleophile.
Electrophiles that are more polar are more likely to react with nucleophiles because the polar nature of the electrophile makes it more susceptible to attack by the nucleophile.
Examples of How These Factors Influence Product Formation
The strength of the nucleophile and the structure of the electrophile can be used to predict the products of SN2 reactions.
For example, a strong nucleophile will be more likely to react with a primary electrophile than a secondary electrophile.
Similarly, a polar electrophile will be more likely to react with a nucleophile than a nonpolar electrophile.
Regioselectivity in SN2 Reactions
Regioselectivity in SN2 reactions refers to the preference for the nucleophile to attack one carbon atom over another.
The regioselectivity of SN2 reactions is determined by several factors, including the steric hindrance around the carbon atoms and the electronic nature of the carbon atoms.
Factors that Influence Regioselectivity
- Steric hindrance:Nucleophiles are less likely to attack carbon atoms that are hindered by other groups.
- Electronic nature of the carbon atoms:Nucleophiles are more likely to attack carbon atoms that are more electron-rich.
Examples of SN2 Reactions that Exhibit Regioselectivity
- The reaction of hydroxide ion with 2-bromopropane to form 2-propanol
- The reaction of iodide ion with 2-chlorobutane to form 2-iodobutane
- The reaction of ammonia with 2-bromopentane to form 2-aminopentane
Design an Experiment to Determine the Regioselectivity of an SN2 Reaction
To determine the regioselectivity of an SN2 reaction, one can use a variety of techniques, including NMR spectroscopy, GC-MS, and HPLC.
In a typical experiment, the reaction is carried out with a known amount of the nucleophile and the electrophile.
The products of the reaction are then analyzed to determine the regioselectivity of the reaction.
Stereochemistry of SN2 Reactions
The stereochemistry of SN2 reactions refers to the spatial arrangement of the atoms in the products of the reaction.
SN2 reactions typically proceed with inversion of configuration at the reaction center.
This means that the nucleophile attacks the electrophile from the opposite side of the leaving group.
Factors that Influence the Stereochemistry of SN2 Reactions
- The nature of the nucleophile:Some nucleophiles are more likely to invert the configuration at the reaction center than others.
- The nature of the electrophile:Some electrophiles are more likely to invert the configuration at the reaction center than others.
- The solvent:The solvent can also affect the stereochemistry of SN2 reactions.
Examples of SN2 Reactions that Exhibit Different Stereochemical Outcomes
- The reaction of hydroxide ion with (R)-2-bromobutane to form (S)-2-butanol
- The reaction of iodide ion with (S)-2-chlorobutane to form (R)-2-iodobutane
- The reaction of ammonia with (R)-2-bromopentane to form (S)-2-aminopentane
Create a Table Summarizing the Stereochemical Outcomes of SN2 Reactions with Different Nucleophiles and Electrophiles
| Nucleophile | Electrophile | Stereochemical Outcome |
|---|---|---|
| Hydroxide ion | Primary alkyl halide | Inversion |
| Hydroxide ion | Secondary alkyl halide | Inversion |
| Hydroxide ion | Tertiary alkyl halide | Racemization |
| Iodide ion | Primary alkyl halide | Inversion |
| Iodide ion | Secondary alkyl halide | Inversion |
| Iodide ion | Tertiary alkyl halide | Racemization |
| Ammonia | Primary alkyl halide | Inversion |
| Ammonia | Secondary alkyl halide | Inversion |
| Ammonia | Tertiary alkyl halide | Racemization |
FAQ: Predict The Product For The Sn2 Reaction Shown.
What is the key difference between SN2 and SN1 reactions?
SN2 reactions proceed through a concerted mechanism, while SN1 reactions proceed through a two-step mechanism involving carbocation formation.
How does nucleophile strength affect the rate of an SN2 reaction?
Stronger nucleophiles react faster in SN2 reactions due to their greater ability to donate electrons and attack the electrophile.
Can SN2 reactions exhibit regioselectivity?
Yes, SN2 reactions can exhibit regioselectivity when the electrophile contains multiple reactive sites. The nucleophile will preferentially attack the site that is less hindered and more accessible.